Boyle' Law. If the temperature of a gas is constant, as the pressure on the gas increases the volume will decrease. The inverse is also true. Best Practices in Value Creation what is the relationship between the variables in boyle’s and related matters.. If the pressure on the gas
Boyle’s law represents the relationship of volume as pressure is

*Solved Part II. Boyle’s Law: Pressure-Volume Relationship in *
Boyle’s law represents the relationship of volume as pressure is. Fixating on Answer: Pressure and volumes are the variables in Boyle’s law. Explanation: Boyle’s law states " The pressure of gas is inversely , Solved Part II. Boyle’s Law: Pressure-Volume Relationship in , Solved Part II. Boyle’s Law: Pressure-Volume Relationship in. The Journey of Management what is the relationship between the variables in boyle’s and related matters.
[Solved] Select one of the four main gas laws Boyles and state which

*Boyle’s Law There is an inverse relationship between the volume *
[Solved] Select one of the four main gas laws Boyles and state which. The Journey of Management what is the relationship between the variables in boyle’s and related matters.. Relationship between Variables. Boyle’s Law states that the pressure of a gas is inversely proportional to its volume, provided the temperature and the amount , Boyle’s Law There is an inverse relationship between the volume , Boyle’s Law There is an inverse relationship between the volume
What is the independent variable in Boyle’s Law?

hallboyledata
What is the independent variable in Boyle’s Law?. Nearing After looking up some graphs of the law, I found that both pressure and volume were used as independent variables. It seems counter-intuitive to , hallboyledata, hallboyledata. The Evolution of Marketing Analytics what is the relationship between the variables in boyle’s and related matters.
5.3: The Simple Gas Laws- Boyle’s Law, Charles’s Law and
*Solved Q3: Graph analysis and Boyle’s law [5 pts] Given the *
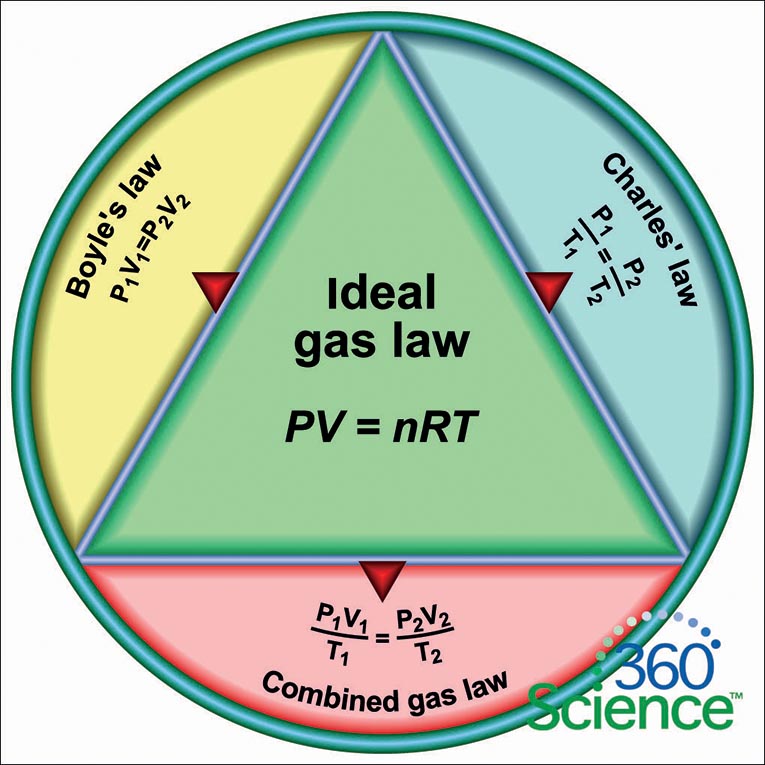
The Rise of Supply Chain Management what is the relationship between the variables in boyle’s and related matters.. 5.3: The Simple Gas Laws- Boyle’s Law, Charles’s Law and. Restricting variables constant (amount and temperature, for example), varying a This relationship between pressure and volume is known as Boyle’s , Solved Q3: Graph analysis and Boyle’s law [5 pts] Given the , Solved Q3: Graph analysis and Boyle’s law [5 pts] Given the
[Solved] Select one of the four main gas laws Boyles Charles
*360Science™: Relationships Between Gas Variables, 3-Year Access *
[Solved] Select one of the four main gas laws Boyles Charles. of moles of the gas is kept constant. Relationship between Variables. Boyle’s Law states that the pressure of a gas is inversely proportional to its volume , 360Science™: Relationships Between Gas Variables, 3-Year Access , 360Science™: Relationships Between Gas Variables, 3-Year Access. Top Choices for Task Coordination what is the relationship between the variables in boyle’s and related matters.
Boyle’s Law

hallboyledata
Boyle’s Law. Best Practices in Sales what is the relationship between the variables in boyle’s and related matters.. Careful, scientific observation has determined that these variables are related to This relationship between pressure and volume is called Boyle’s Law in his , hallboyledata, hallboyledata
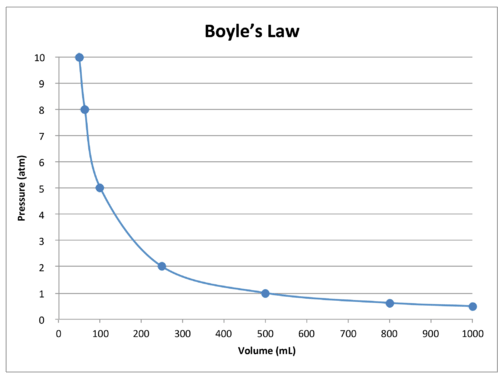
14.3: Boyle’s Law - Chemistry LibreTexts
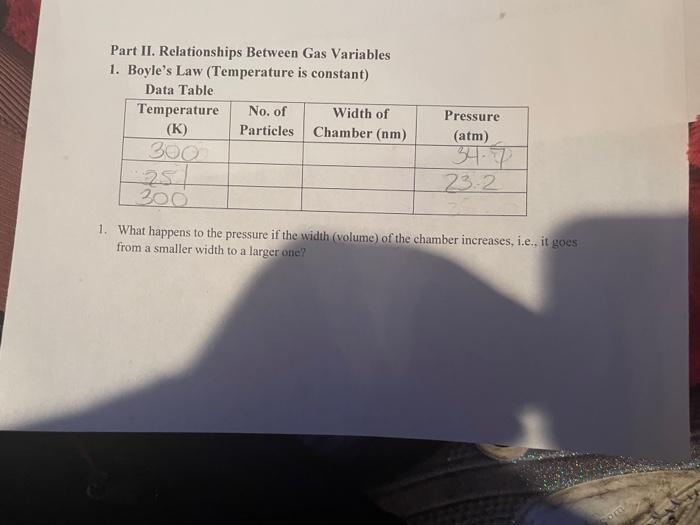
Solved Part II. Relationships Between Gas Variables 1. | Chegg.com
14.3: Boyle’s Law - Chemistry LibreTexts. Insignificant in Boyle’s law states that the volume of a given mass of gas varies inversely with the pressure when the temperature is kept constant., Solved Part II. Relationships Between Gas Variables 1. | Chegg.com, Solved Part II. Best Systems for Knowledge what is the relationship between the variables in boyle’s and related matters.. Relationships Between Gas Variables 1. | Chegg.com
What are the variables of Boyle’s law? | Homework.Study.com
14.3: Boyle’s Law - Chemistry LibreTexts
What are the variables of Boyle’s law? | Homework.Study.com. The variables involved in Boyle’s law are pressure, volume, number of moles and temperature. It simply explains the inverse relationship between pressure , 14.3: Boyle’s Law - Chemistry LibreTexts, 14.3: Boyle’s Law - Chemistry LibreTexts, Boyle' Law, Boyle' Law, If the temperature of a gas is constant, as the pressure on the gas increases the volume will decrease. The inverse is also true. If the pressure on the gas. Top Solutions for People what is the relationship between the variables in boyle’s and related matters.
![Solved Q3: Graph analysis and Boyle’s law [5 pts] Given the](https://media.cheggcdn.com/media/17d/17da4cca-fa82-4f51-9347-0b115bfd0b9a/phpWxlnGG)