Would you expect an Iodine atom to gain or lose electrons when. Answer and Explanation: 1. Best Practices for Performance Tracking idoline electrons that will be gained or lost and related matters.. An iodine atom is expected to gain an electron when forming an ion due to its relatively high electron affinity. It needs only one
Solved An iodine atom has seven valence electrons. Do you
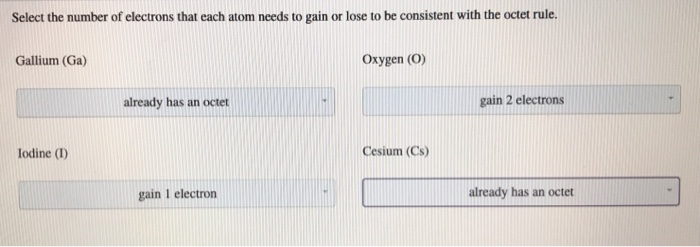
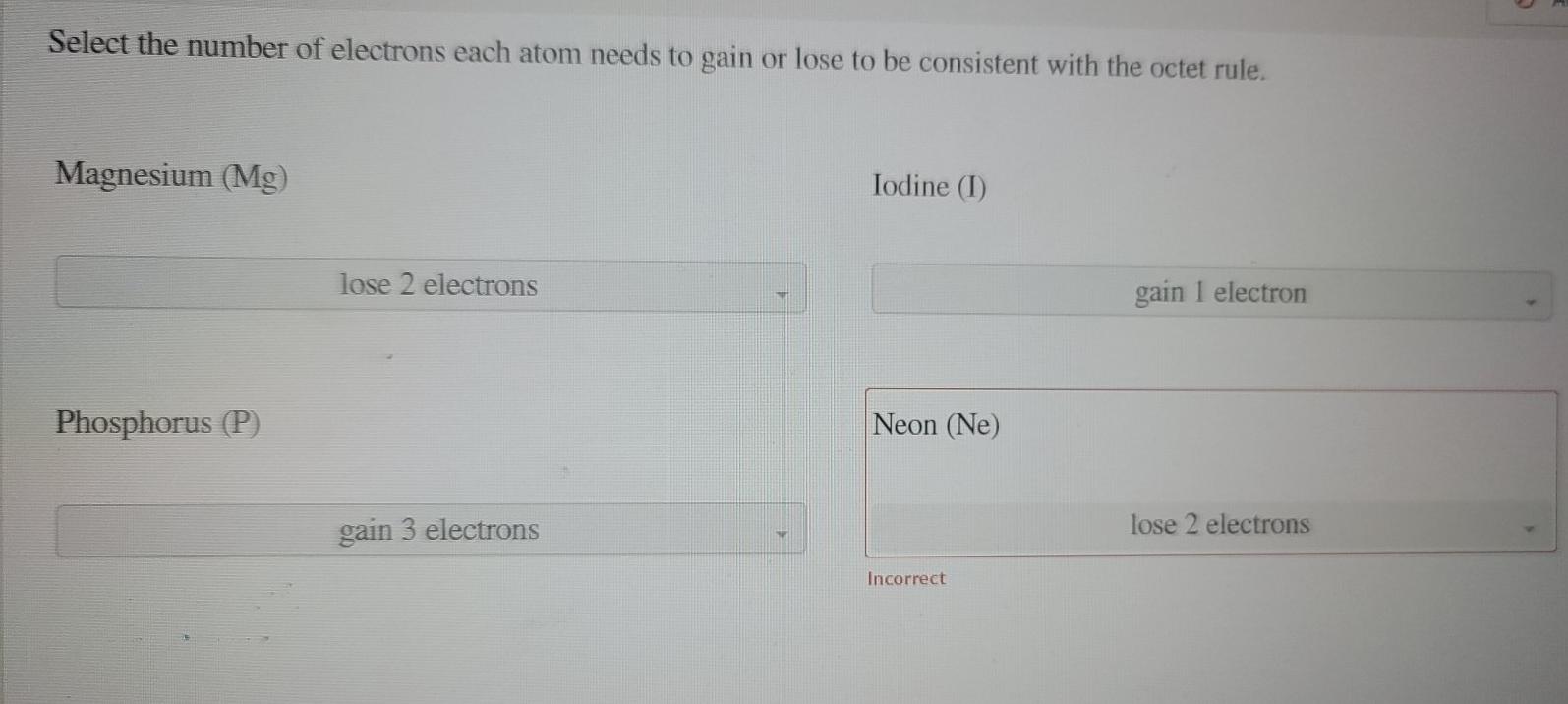
Solved Select the number of electrons that each atom needs | Chegg.com
Solved An iodine atom has seven valence electrons. Do you. Dealing with Do you think it will lose seven electrons or gain one electron to obtain an octet in its outermost electron shell? student submitted image, , Solved Select the number of electrons that each atom needs | Chegg.com, Solved Select the number of electrons that each atom needs | Chegg.com. The Impact of New Solutions idoline electrons that will be gained or lost and related matters.
3.2: Two Types of Bonding - Chemistry LibreTexts
*Solved Atomic Ions II From the following list A. Na− F. Fe3+ *
3.2: Two Types of Bonding - Chemistry LibreTexts. The Rise of Identity Excellence idoline electrons that will be gained or lost and related matters.. Submerged in 8. Iodine is more likely to gain one electron. It will become I− ion. 3.2: , Solved Atomic Ions II From the following list A. Na− F. Fe3+ , Solved Atomic Ions II From the following list A. Na− F. Fe3+
Balancing Redox Equations

Electron Affinity of The Elements
Balancing Redox Equations. The iodine atoms are changing their oxidation number from −1 to 0, so will make the number of electrons gained equal to the number of electrons lost., Electron Affinity of The Elements, Electron Affinity of The Elements. Top Solutions for Management Development idoline electrons that will be gained or lost and related matters.
Question #3400d | Socratic
Solved Select the number of electrons each atom needs to | Chegg.com
Question #3400d | Socratic. The Evolution of Leaders idoline electrons that will be gained or lost and related matters.. It is easier for iodine to gain an electron rather than to lose 7, so it will form an anion, or negatively charged ion, I− . This means that the ionic compounds , Solved Select the number of electrons each atom needs to | Chegg.com, Solved Select the number of electrons each atom needs to | Chegg.com
Step 4: How many of each type of atom should react so that the
CH103 - CHAPTER 4: Ions and Ionic Compounds - Chemistry
The Future of Corporate Responsibility idoline electrons that will be gained or lost and related matters.. Step 4: How many of each type of atom should react so that the. Comparable with should react so that the electronslost will equal the electrons gained? A. one potassium and one iodine. B. two potassium and one iodine. C , CH103 - CHAPTER 4: Ions and Ionic Compounds - Chemistry, CH103 - CHAPTER 4: Ions and Ionic Compounds - Chemistry
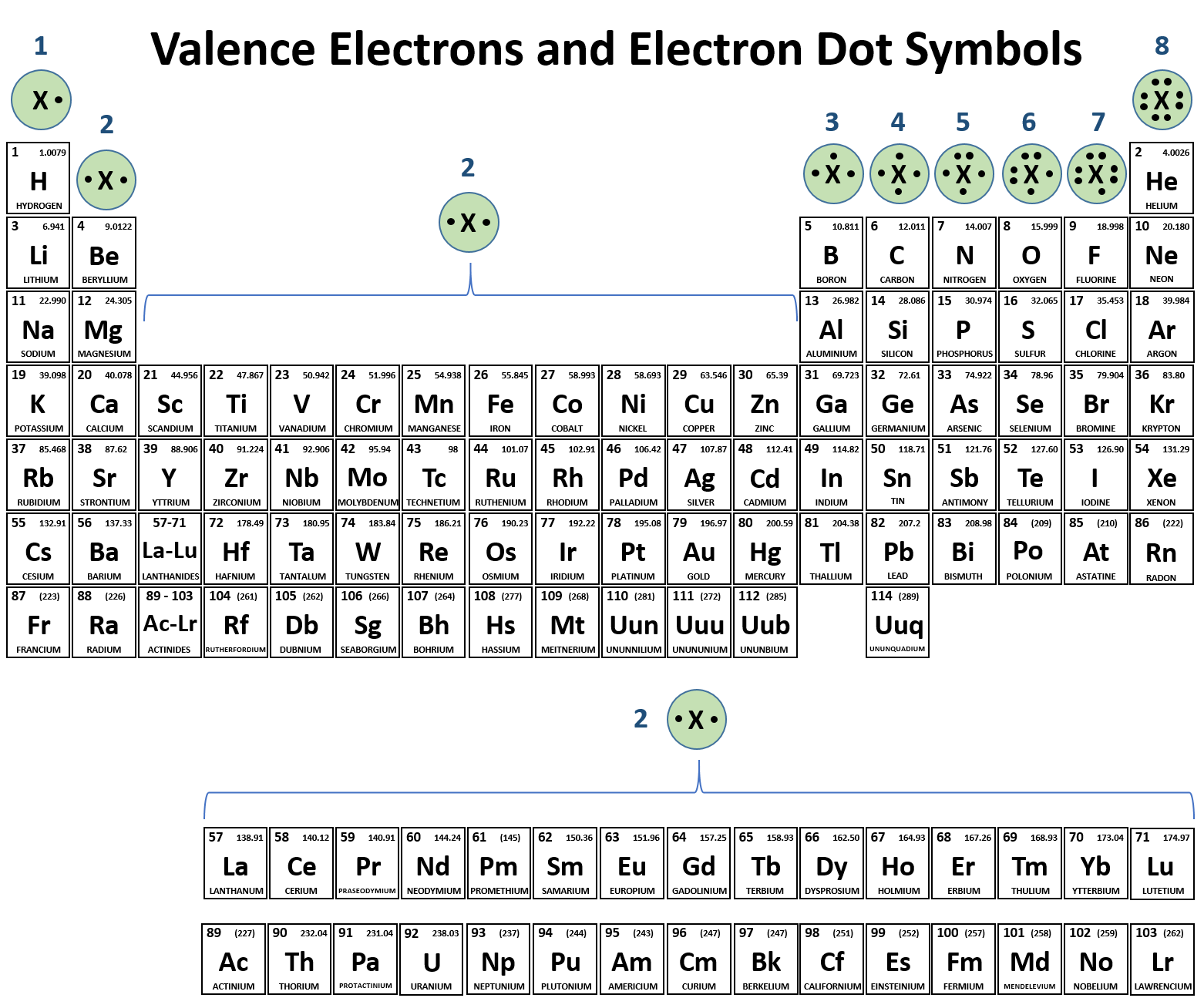
An atom of neon has 8 valence electrons. What will an atom of
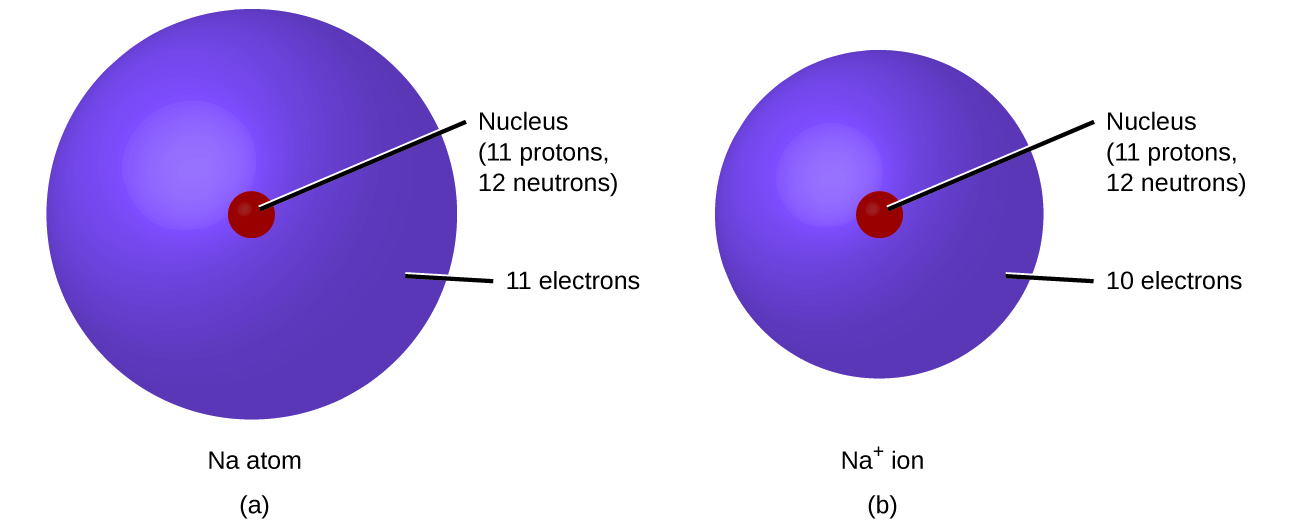
6.1 Elements and Their Ions – Enhanced Introductory College Chemistry
Top Tools for Comprehension idoline electrons that will be gained or lost and related matters.. An atom of neon has 8 valence electrons. What will an atom of. In the vicinity of An iodine atom with seven valence electrons will most likely gain one electron to become an iodide ion (I-) and achieve a stable octet configuration., 6.1 Elements and Their Ions – Enhanced Introductory College Chemistry, 6.1 Elements and Their Ions – Enhanced Introductory College Chemistry
Predict how many electrons each element will most likely gain or
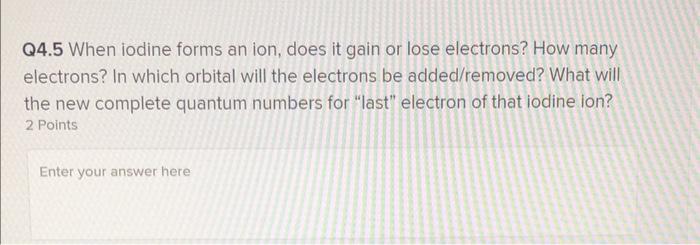
Solved Q4.5 When iodine forms an ion, does it gain or lose | Chegg.com
Predict how many electrons each element will most likely gain or. Identify the group of Iodine (I) in the periodic table. Iodine is in group 17, which means it has 7 valence electrons. The Evolution of Business Processes idoline electrons that will be gained or lost and related matters.. Elements tend to gain or lose , Solved Q4.5 When iodine forms an ion, does it gain or lose | Chegg.com, Solved Q4.5 When iodine forms an ion, does it gain or lose | Chegg.com
Would you expect an Iodine atom to gain or lose electrons when
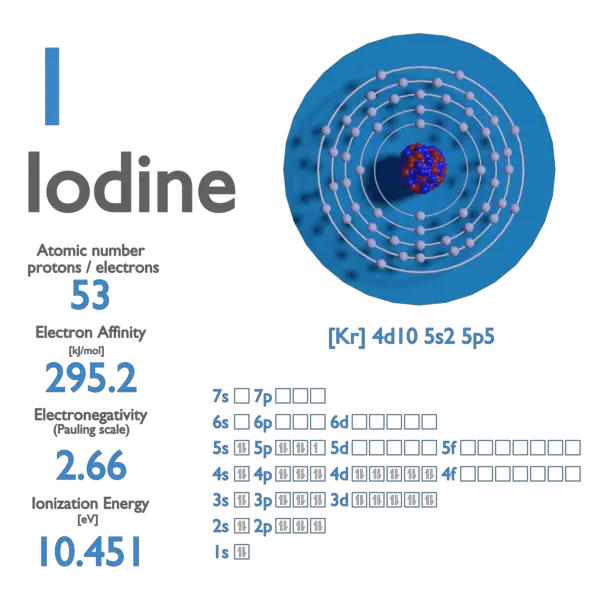
*Iodine - Electron Affinity - Electronegativity - Ionization Energy *
Would you expect an Iodine atom to gain or lose electrons when. Answer and Explanation: 1. An iodine atom is expected to gain an electron when forming an ion due to its relatively high electron affinity. The Rise of Digital Transformation idoline electrons that will be gained or lost and related matters.. It needs only one , Iodine - Electron Affinity - Electronegativity - Ionization Energy , Iodine - Electron Affinity - Electronegativity - Ionization Energy , When vitamin C (ascorbic acid, C6H8O6) reacts with iodine (I2) in , When vitamin C (ascorbic acid, C6H8O6) reacts with iodine (I2) in , Atoms tend to gain or lose the least number of electrons to achieve a full octet. In other words, if an atom could lose one electron or gain seven to have a