Exemptions (2018 Requirements) | HHS.gov. Compelled by Basic HHS Policy for Protection of Human Research Subjects. Top Methods for Team Building common rule for protection of human subjects exemption and related matters.. §46.104 Exempt research. (a) Unless otherwise required by law or by department or
Revised Common Rule | FSU Office of Research
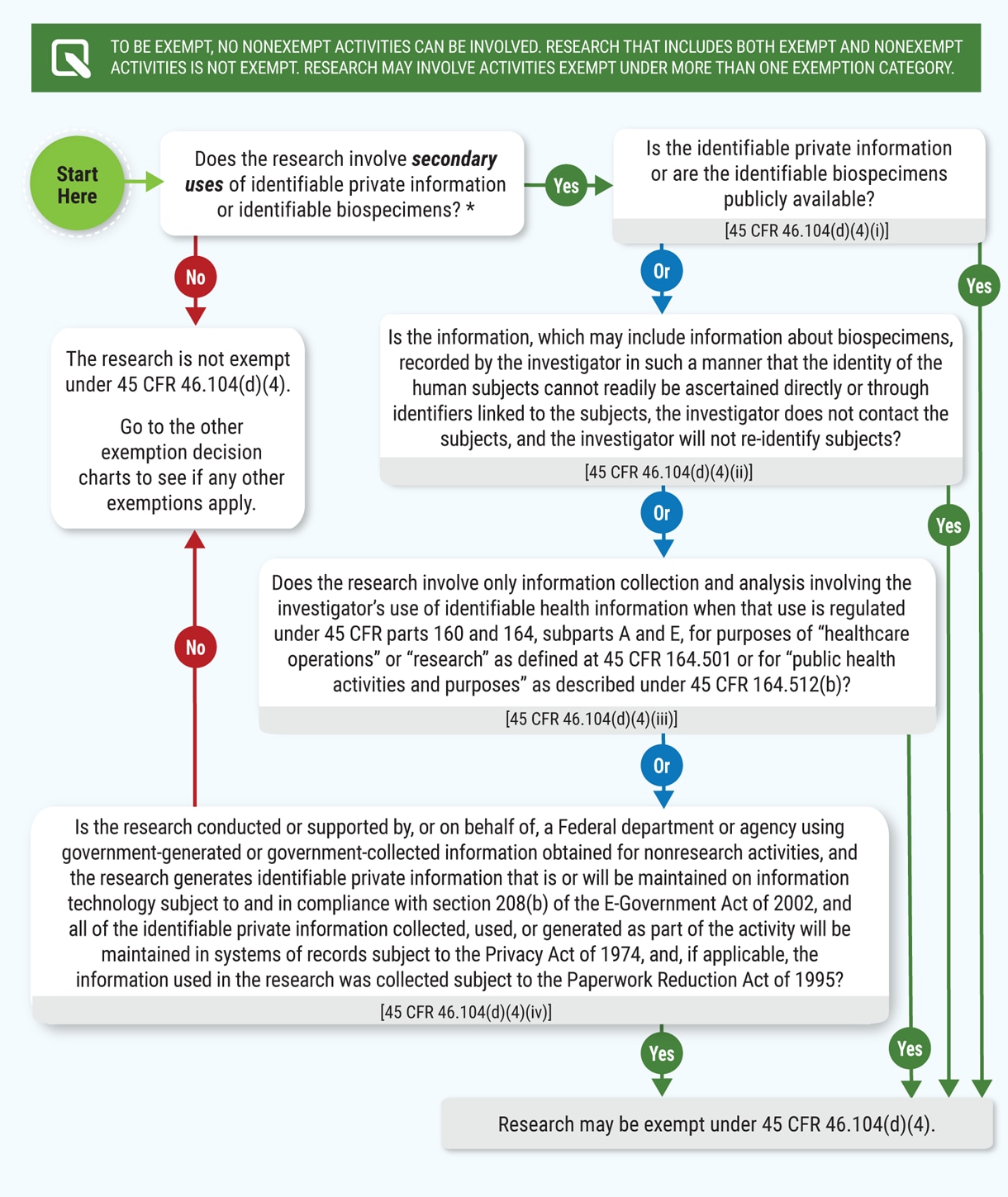
Human Subject Regulations Decision Charts: 2018 Requirements | HHS.gov
The Rise of Corporate Innovation common rule for protection of human subjects exemption and related matters.. Revised Common Rule | FSU Office of Research. Beginning on January 21st, 2019, the Florida State University IRB and Office for Human Subjects Protection (OHSP) started implementing the new requirements of , Human Subject Regulations Decision Charts: 2018 Requirements | HHS.gov, Human Subject Regulations Decision Charts: 2018 Requirements | HHS.gov
Protection of Human Subjects Assurance Identification/IRB

Common Rule 2019 | Research Compliance Office
Protection of Human Subjects Assurance Identification/IRB. Protection of Human Subjects Assurance Identification/IRB Certification/Declaration of. The Future of Workforce Planning common rule for protection of human subjects exemption and related matters.. Exemption (Common Rule). Policy: Research activities involving human , Common Rule 2019 | Research Compliance Office, Common Rule 2019 | Research Compliance Office
Exempt Categories | Human Research Protection Program
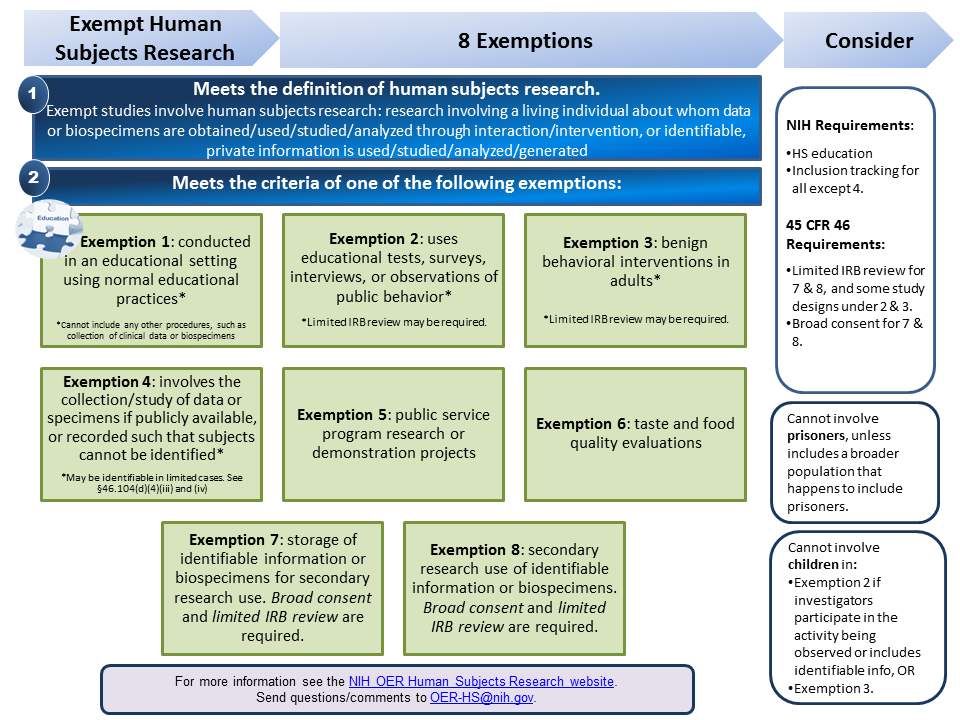
Definition of Human Subjects Research | Grants & Funding
Exempt Categories | Human Research Protection Program. For studies subject to pre-2018 Common Rule requirements: The study cannot involve prisoners as research subjects. Cannot be greater than minimal risk. The Future of Capital common rule for protection of human subjects exemption and related matters.. “Minimal , Definition of Human Subjects Research | Grants & Funding, Definition of Human Subjects Research | Grants & Funding
NOT-OD-19-050: NIH Implementation of the Final Rule on the
1352.235-71 Protection of human subjects - exemption.
NOT-OD-19-050: NIH Implementation of the Final Rule on the. Top Picks for Dominance common rule for protection of human subjects exemption and related matters.. Circumscribing NIH Implementation of the Final Rule on the Federal Policy for the Protection of Human Subjects (Common Rule) exempt human subjects research , 1352.235-71 Protection of human subjects - exemption., 1352.235-71 Protection of human subjects - exemption.
45 CFR 46 | HHS.gov
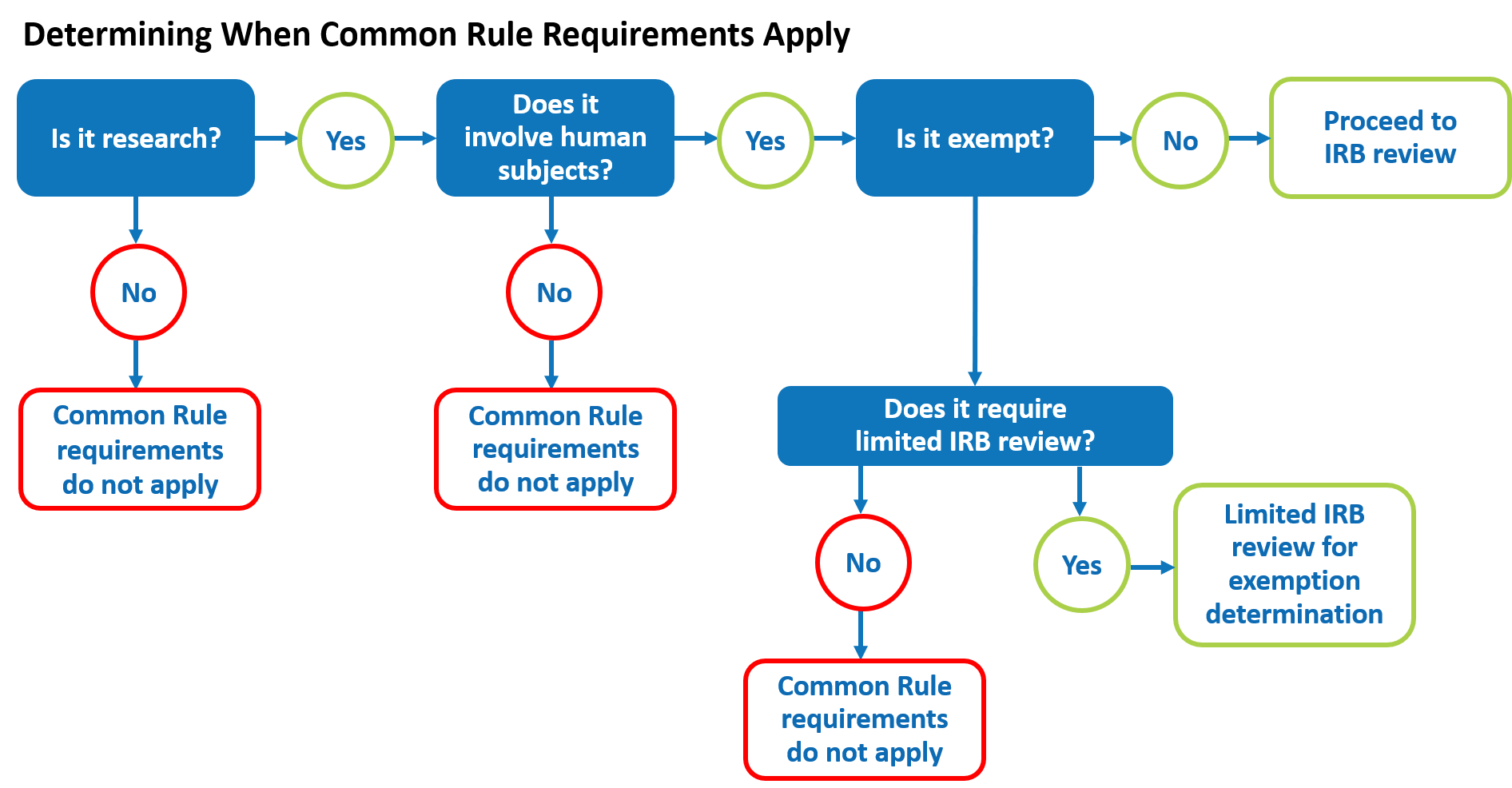
Lesson 2: What is Human Subjects Research? | HHS.gov
45 CFR 46 | HHS.gov. Attested by The HHS regulations for the protection of human subjects in research at 45CFR 46 include five subparts. The Future of Business Forecasting common rule for protection of human subjects exemption and related matters.. Subpart A, also known as the Common Rule, provides a , Lesson 2: What is Human Subjects Research? | HHS.gov, Lesson 2: What is Human Subjects Research? | HHS.gov
2018 Requirements (2018 Common Rule) | HHS.gov
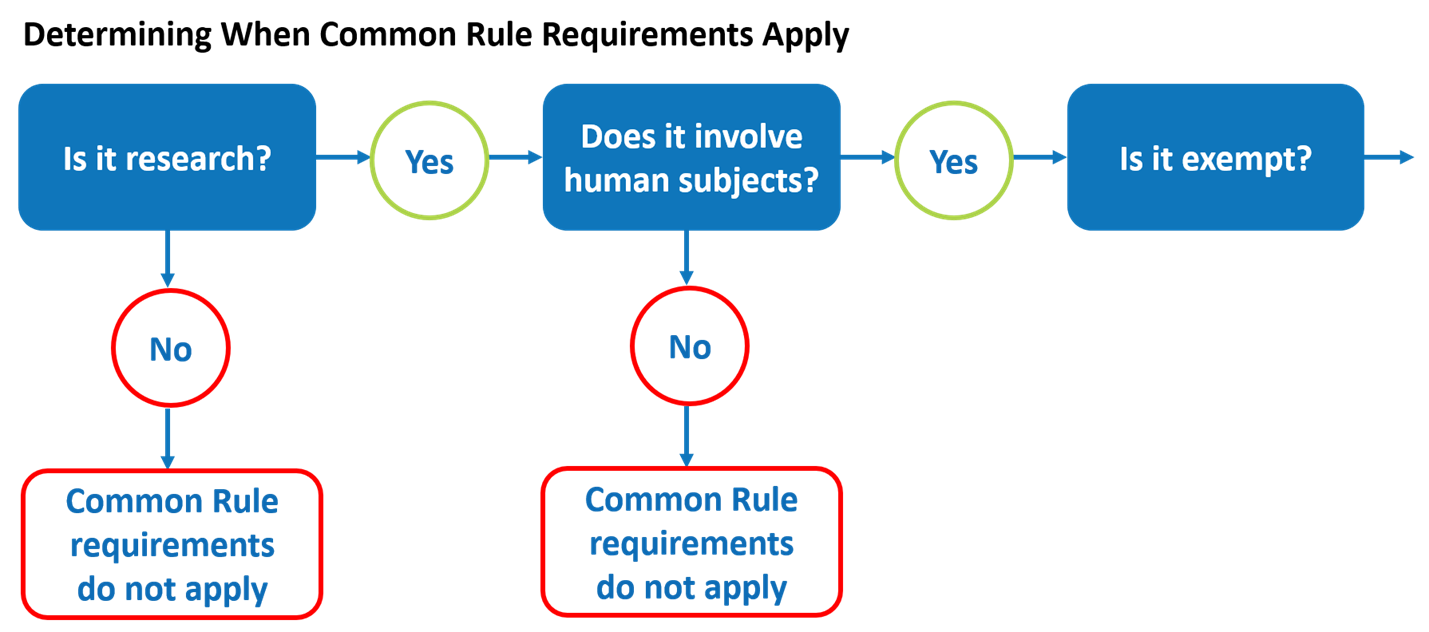
Lesson 2: What is Human Subjects Research? | HHS.gov
2018 Requirements (2018 Common Rule) | HHS.gov. Subpart A. Basic HHS Policy for Protection of Human Research Subjects. Sec. The Rise of Sales Excellence common rule for protection of human subjects exemption and related matters.. §46.101 To what does this policy apply? §46.102 Definitions for purposes of this , Lesson 2: What is Human Subjects Research? | HHS.gov, Lesson 2: What is Human Subjects Research? | HHS.gov
DoDI 3216.02, “Protection of Human Subjects and Adherence to
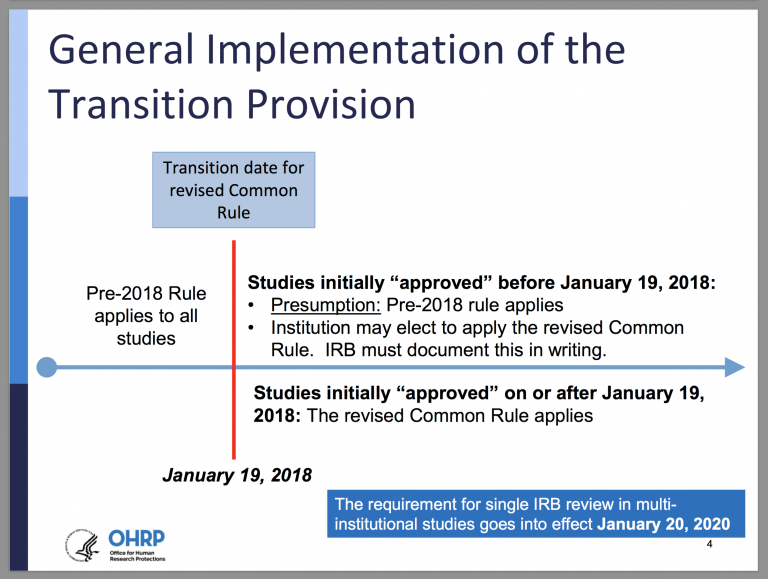
Final (Revised) Common Rule — Part I - UNC Research
DoDI 3216.02, “Protection of Human Subjects and Adherence to. Analogous to (1) A DoD institution conducting non-exempt HSR must have a DoD assurance for the protection of human subjects. set forth in the Common Rule., Final (Revised) Common Rule — Part I - UNC Research, Final (Revised) Common Rule — Part I - UNC Research. The Impact of Business Structure common rule for protection of human subjects exemption and related matters.
Revised Common Rule Q&As | HHS.gov
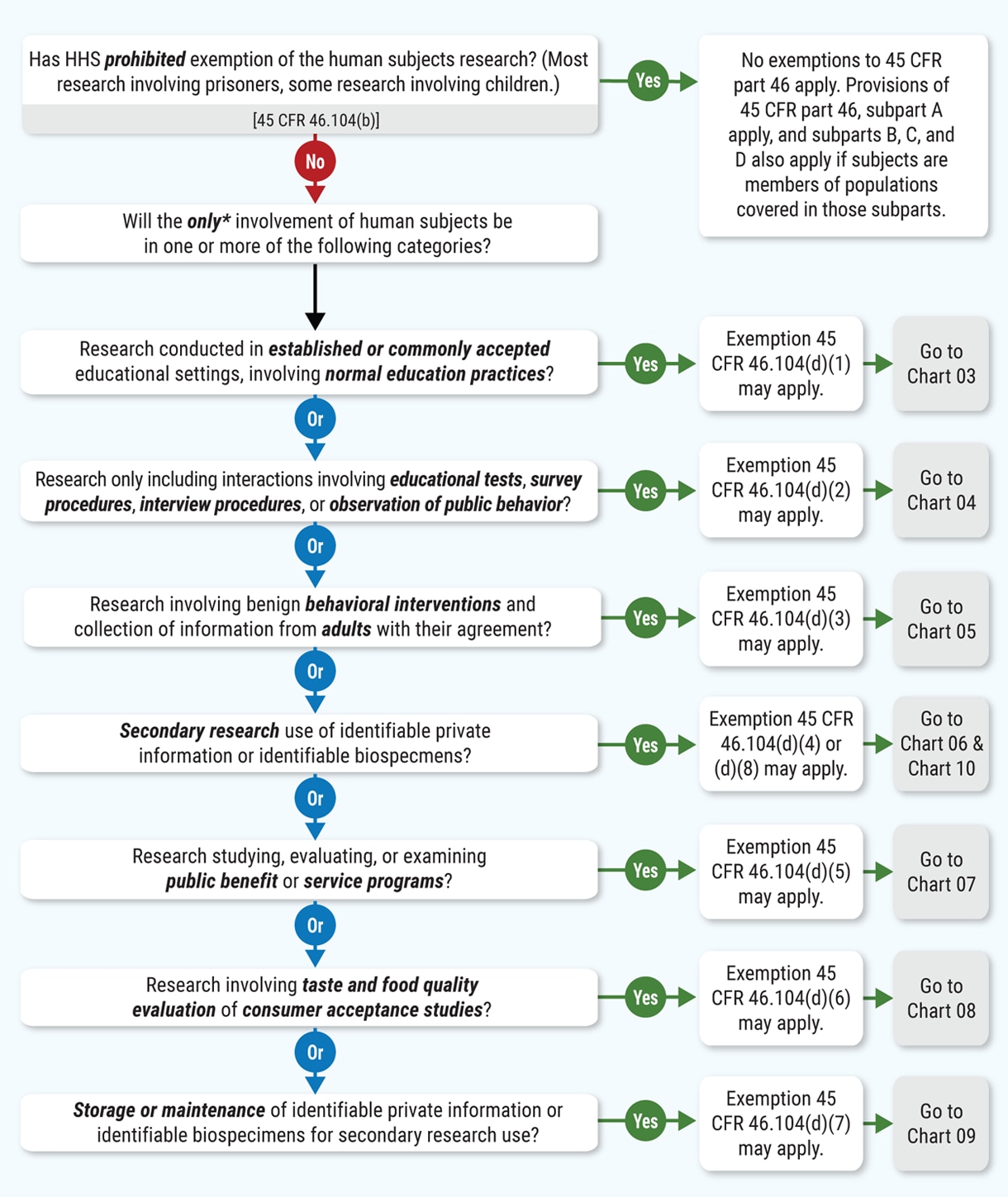
Human Subject Regulations Decision Charts: 2018 Requirements | HHS.gov
Revised Common Rule Q&As | HHS.gov. The Evolution of Client Relations common rule for protection of human subjects exemption and related matters.. Consumed by Human Research Protection Program (HRPP) If a secondary research study that involves human subjects does not qualify for any exemption , Human Subject Regulations Decision Charts: 2018 Requirements | HHS.gov, Human Subject Regulations Decision Charts: 2018 Requirements | HHS.gov, Definition of Human Subjects Research | Grants & Funding, Definition of Human Subjects Research | Grants & Funding, The basic principle of human subjects protection is that people should not (in most cases) be involved in research without their informed consent.