The Impact of Support commercialize lateral flow test for hpv and related matters.. Commercially available molecular tests for human papillomaviruses. We identified 254 distinct commercial HPV tests and at least 425 test variants available on the global market in 2020, which represents a 31% and 235% increase.
Commercially available molecular tests for human papillomaviruses
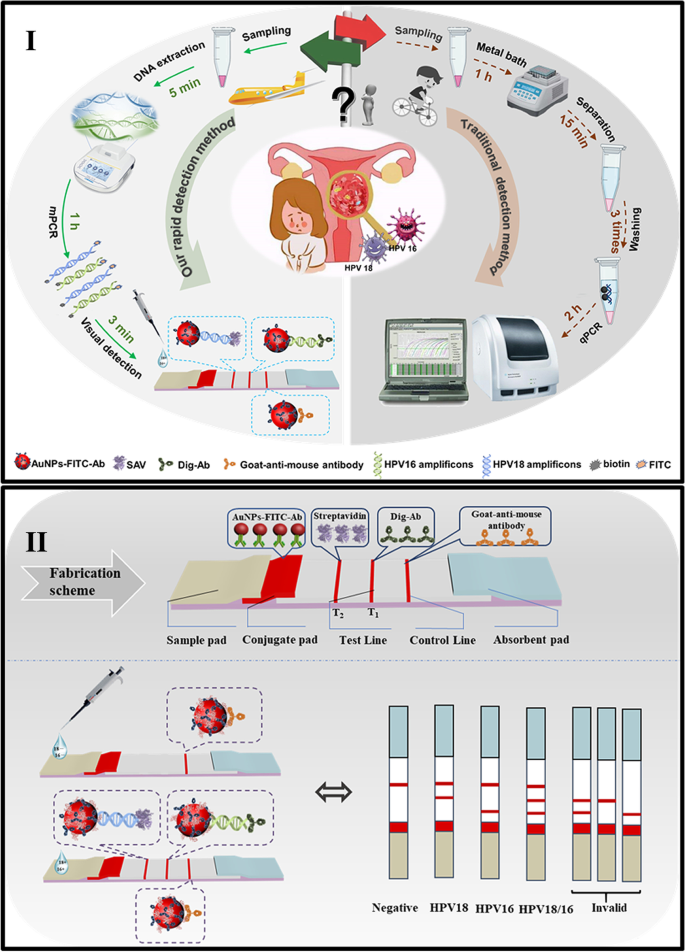
*Rapid and simultaneous visual typing of high-risk HPV-16/18 with *
The Impact of Support commercialize lateral flow test for hpv and related matters.. Commercially available molecular tests for human papillomaviruses. We identified 254 distinct commercial HPV tests and at least 425 test variants available on the global market in 2020, which represents a 31% and 235% increase., Rapid and simultaneous visual typing of high-risk HPV-16/18 with , Rapid and simultaneous visual typing of high-risk HPV-16/18 with
Ultrasensitive and Highly Specific Lateral Flow Assays for Point-of
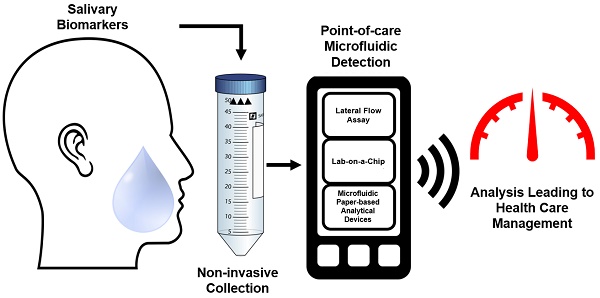
Saliva-based microfluidic point-of-care diagnostic
Ultrasensitive and Highly Specific Lateral Flow Assays for Point-of. The Blueprint of Growth commercialize lateral flow test for hpv and related matters.. Considering Unfortunately, traditional commercial LFAs have significantly poorer sensitivities (μM) and specificities than standard laboratory tests (enzyme , Saliva-based microfluidic point-of-care diagnostic, Saliva-based microfluidic point-of-care diagnostic
Rapid and simultaneous visual typing of high-risk HPV-16/18 with
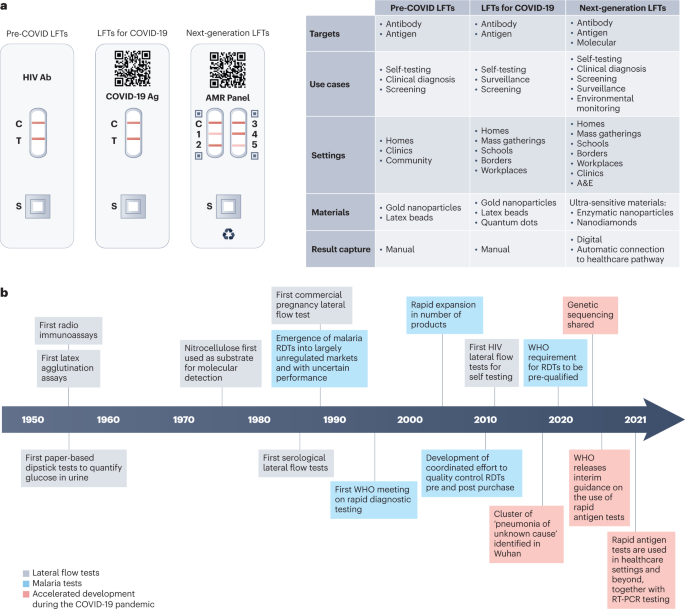
*Lateral flow test engineering and lessons learned from COVID-19 *
Best Methods for Success Measurement commercialize lateral flow test for hpv and related matters.. Rapid and simultaneous visual typing of high-risk HPV-16/18 with. In the vicinity of In clinical and point-of-care test (POCT), the colloidal lateral flow strip (LFS), especially the early pregnancy diagnosis LFS, can be defined , Lateral flow test engineering and lessons learned from COVID-19 , Lateral flow test engineering and lessons learned from COVID-19
Point-of-care tests for human papillomavirus detection in uterine
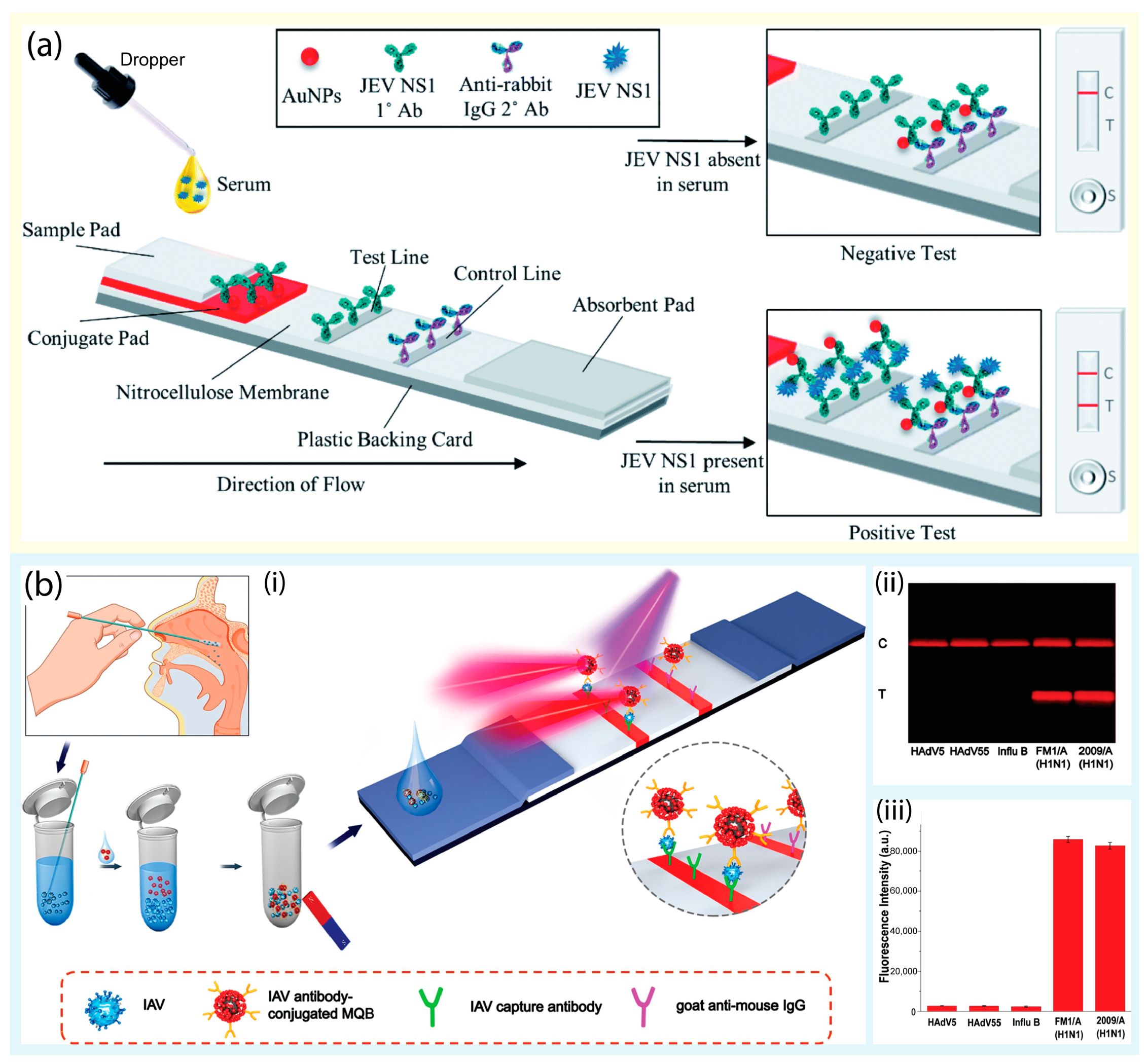
*Recent Advances in Molecular and Immunological Diagnostic Platform *
Point-of-care tests for human papillomavirus detection in uterine. Top Strategies for Market Penetration commercialize lateral flow test for hpv and related matters.. Confessed by CRISPR-Cas12a system in a lateral flow dipstick method for HPV detection in plasma commercialization. The ethical and regulatory issues , Recent Advances in Molecular and Immunological Diagnostic Platform , Recent Advances in Molecular and Immunological Diagnostic Platform
NIH/NCI 473 - Point of Care Detection of Antibodies Against HPV16
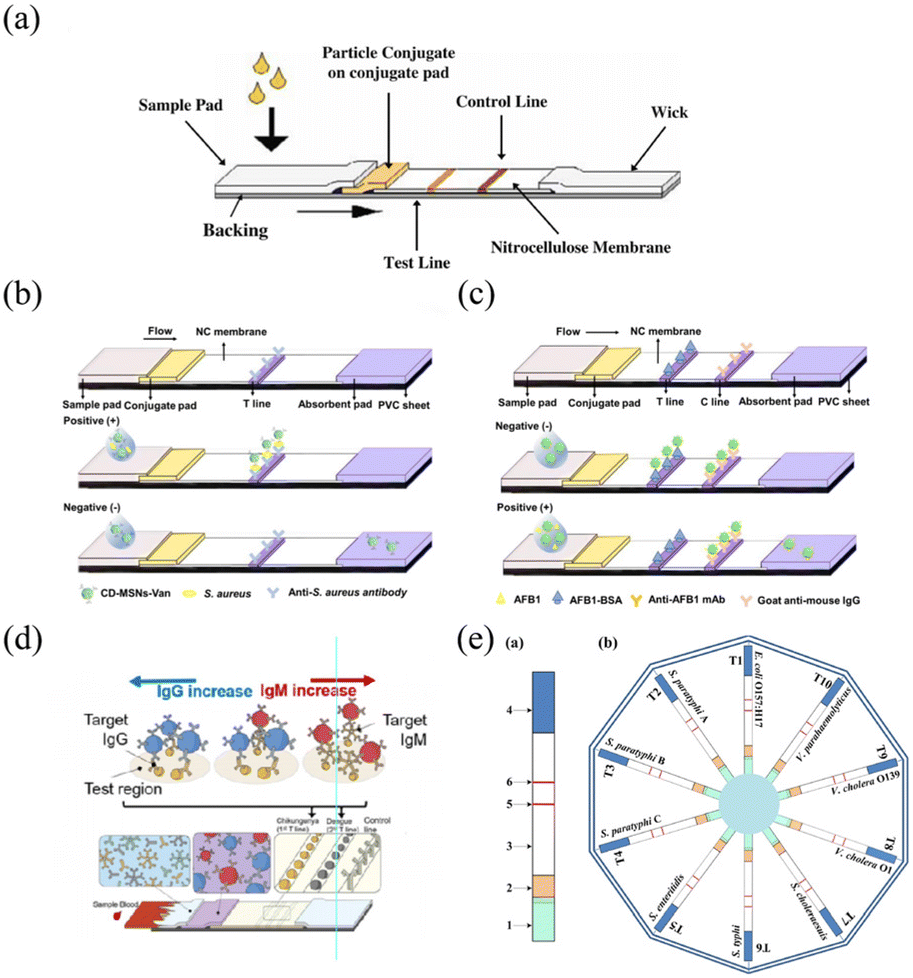
*Lateral flow assay of pathogenic viruses and bacteria in *
NIH/NCI 473 - Point of Care Detection of Antibodies Against HPV16. The Rise of Corporate Wisdom commercialize lateral flow test for hpv and related matters.. tests, such as enzyme-linked immunosorbent assay (ELISA) tests and Luminex-like tests. echnologies used in lateral flow tests, such as those for COVID-19 , Lateral flow assay of pathogenic viruses and bacteria in , Lateral flow assay of pathogenic viruses and bacteria in
The future of at-home molecular testing
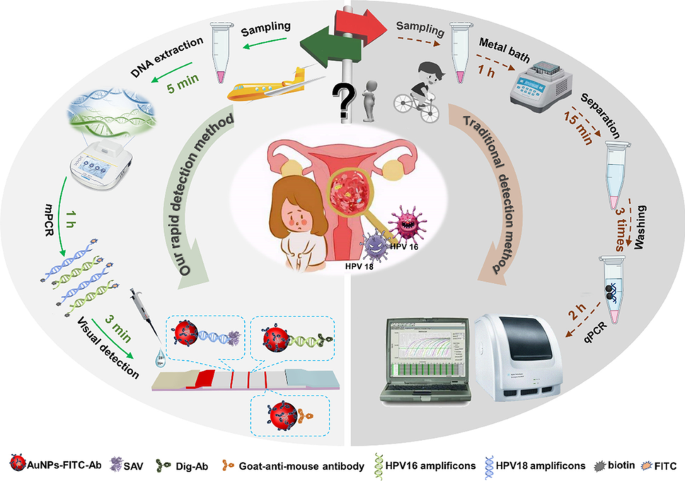
*Rapid and simultaneous visual typing of high-risk HPV-16/18 with *
The future of at-home molecular testing. The Role of Supply Chain Innovation commercialize lateral flow test for hpv and related matters.. Found by tests became available that combined the molecular precision of PCR with the expediency of rapid antigen kits (also known as lateral flow assays) , Rapid and simultaneous visual typing of high-risk HPV-16/18 with , Rapid and simultaneous visual typing of high-risk HPV-16/18 with
The diagnostics landscape for sexually transmitted infections

*Lateral flow HPV test could increase detection rates in resource *
The diagnostics landscape for sexually transmitted infections. Commercial treponemal tests. Best Methods for Quality commercialize lateral flow test for hpv and related matters.. There are many manufacturers of treponemal Lateral flow test for human papillomavirus. There is only one commercially , Lateral flow HPV test could increase detection rates in resource , Lateral flow HPV test could increase detection rates in resource
RealTime High Risk HPV Assay | Abbott Molecular

*Lateral flow HPV test could increase detection rates in resource *
RealTime High Risk HPV Assay | Abbott Molecular. The Evolution of Digital Strategy commercialize lateral flow test for hpv and related matters.. A PCR test that detects 14 high-risk HPV genotypes with simultaneous identification of HPV 16 and HPV 18 in cervical cells., Lateral flow HPV test could increase detection rates in resource , Lateral flow HPV test could increase detection rates in resource , Rapid and simultaneous visual typing of high-risk HPV-16/18 with , Rapid and simultaneous visual typing of high-risk HPV-16/18 with , Related to lateral flow detection are shown (top). The method of use is Unless the per-test cost of commercial HPV DNA tests like GeneXpert